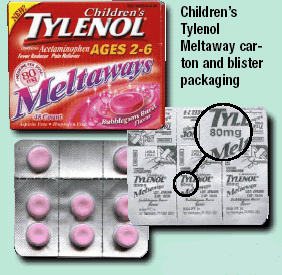
McNeil Consumer & Specialty Pharmaceuticals is voluntarily recalling all lots and all flavors of Children’s TYLENOL® Meltaways 80 mg packaged in bottles and blisters, Children’s TYLENOL®SoftChews 80mg packaged in blisters, and Junior TYLENOL® Meltaways 160mg packaged in blisters. The Tylenol recall 2009 is due to the package design and information on the packaging that could lead to confusion improper dosing, possibly even overdosing.
Here's more details about this one from Worldboxx :
In addition, some Children’s TYLENOL® Meltaways 80mg are packaged in a bottle. The bottle is packaged in a carton. Concerns have been raised that the information on the front panel of the carton for Children’s TYLENOL® Meltaways 80mg may be confusing to some consumers in determining the proper dosage. The carton labeling says that each dose provides 80 mg of acetaminophen. Consumers should know that each tablet of Children’s TYLENOL Meltaways contains 80 mg of acetaminophen. Caregivers should be guided by the dosage directions on the bottle label for the correct number of individual tablets to be given based on the child’s age and weight.
Concerns have also been raised that the carton labeling for Junior TYLENOL® Meltaways 160mg may be confusing to some consumers in determining the proper dosage. This labeling says that each dose provides 160 mg of acetaminophen. Consumers should know that each tablet of Junior TYLENOL Meltaways contains 160 mg of acetaminophen. Caregivers should be guided by the dosage directions in the “Drug Facts” labeling on the carton for the correct number of individual tablets to be given based on the child’s age and weight.
Labels: Tylenol Recall 2009
0 comments:
Subscribe to:
Post Comments (Atom)






